BTK Inhibitor
Pirtobrutinib
Related Resources:
Privacy Preferences
Lilly collects information about your online interactions, such as user ID, browsing history, geolocation and IP address, through tracking technologies, such as third-party cookies..... You may accept, decline or withdraw your consent to most cookies and tracking technologies on this site, other than those that are necessary for the site operations and functionality, by clicking one of the options below. We use this information to personalize content, save your preferences, and track website performance. We share information with our analytics, marketing, and advertising partners for purposes of targeted advertising and analyzing website metrics. Please visit our Cookie Notice, Privacy Statement, and Consumer Health Privacy Notice for more information. Certain laws require consent to collect and share information. You can withdraw your consent at any time through the Cookie Settings or Your Privacy Choices in our website footer. View More
Users located in Washington or Nevada must use this link to Decline All
We have detected a Global Privacy Control (GPC) signal, please confirm if you wish to accept all cookies including or excluding those flagged as GPC.
Manage Your Cookie Settings
For more information about Lilly's cookies, tracking technologies and third-party cookies, see our Cookie Notice.
You appear to be in a location where some cookies cannot be enabled.
Always On
These cookies are necessary for the website to function properly and cannot be switched off in our systems. Strictly necessary cookies cannot be disabled. For example, these cookies may enable us to optimize page and content loading, distribute and manage site traffic, detect bugs and errors and block malicious traffic and suspicious activity. In addition, these cookies may enable third party-supported features that you use, such as video players and chat tools, and other key site functionality, such as webforms.
Strictly Necessary Cookie
These cookies and similar technologies remember choices you make such as language or search parameters. We use these cookies to improve your user experience and to make your use of the websites more tailored. If you do not allow these cookies, then some or all of these services may not function as designed.
These cookies and similar technologies remember choices you make such as language or search parameters. We use these cookies to improve your user experience and to make your use of the websites more tailored. If you do not allow these cookies, then some or all of these services may not function as designed.
These cookies allow Lilly to analyze visits and traffic sources so we can measure and improve the performance of our sites. They help us to know which pages are the most and least popular, and see how visitors move around the site to improve the site’s performance.
These cookies allow Lilly to analyze visits and traffic sources so we can measure and improve the performance of our sites. They help us to know which pages are the most and least popular, and see how visitors move around the site to improve the site’s performance.
These cookies and similar technologies enable our advertising and marketing partners to provide behavioral advertising and use functionalities like social media pixels to track your online activity across social platforms and websites, which can identify you, as described in the Privacy Statement and Consumer Health Privacy Notice. These methods create a profile of your activity for our business and commercial purposes and enable Lilly and our advertising partners to customize offers and information (online or in person) to you based on your profile, and profiles of persons with similar interests, that may enhance your interactions with Lilly.
These cookies and similar technologies enable our advertising and marketing partners to provide behavioral advertising and use functionalities like social media pixels to track your online activity across social platforms and websites, which can identify you, as described in the Privacy Statement and Consumer Health Privacy Notice. These methods create a profile of your activity for our business and commercial purposes and enable Lilly and our advertising partners to customize offers and information (online or in person) to you based on your profile, and profiles of persons with similar interests, that may enhance your interactions with Lilly.

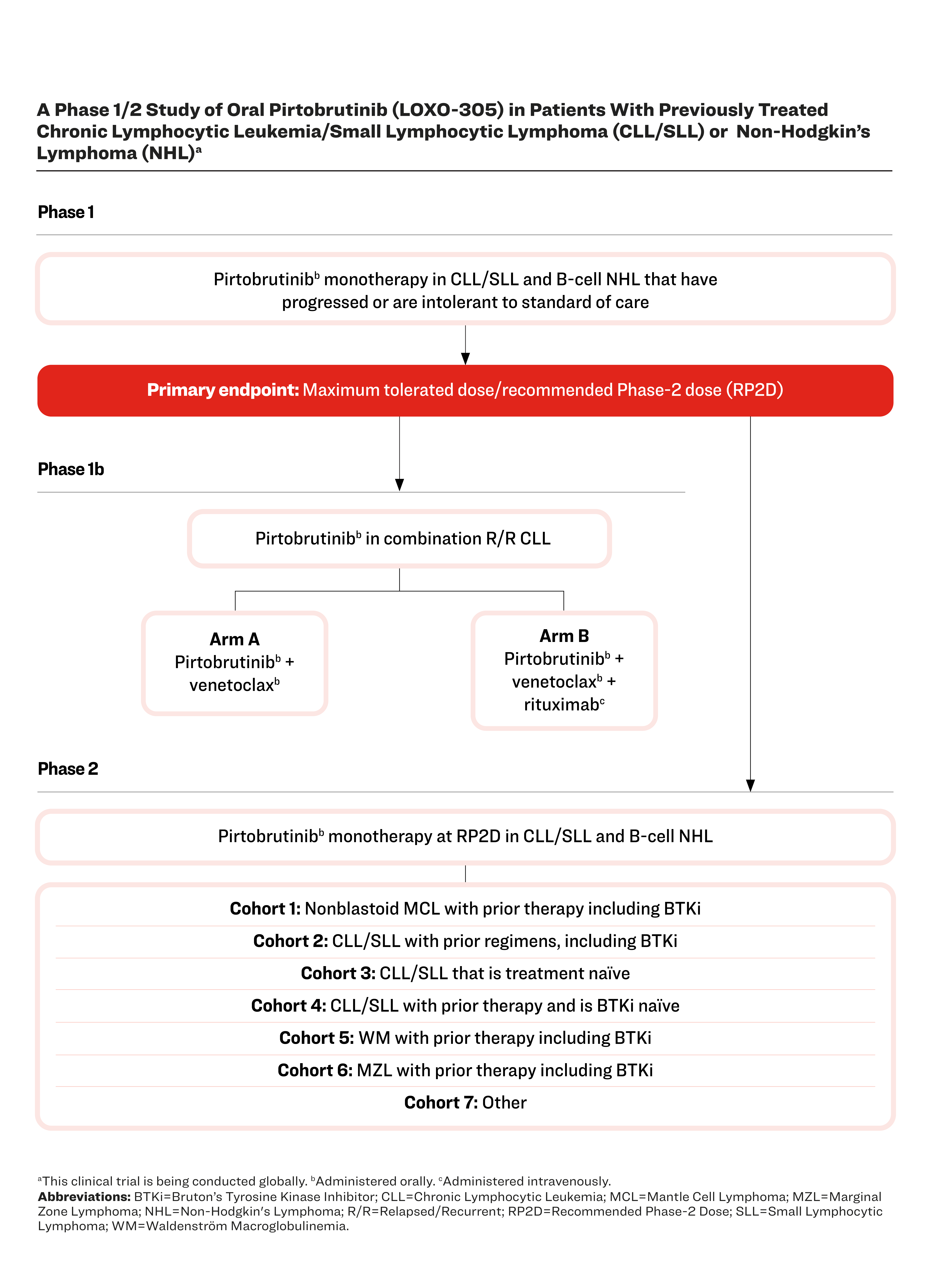
or visit www.clinicaltrials.gov for more information on this trial
This website was commissioned by Lilly Medical and is indicated to be used by HCPs for medical, scientific, and educational purposes.
The safety and efficacy of the agents for uses under investigation have not been established. Pipeline molecules may not receive regulatory approval and become commercially available for the uses being investigated. The information provided about new molecules being studied is for scientific information exchange purposes only with no commercial intent.
This information is current through March 2025.
VV-OTHR-US-DEL-4476 03/2025 © 2025 Lilly USA, LLC. All rights reserved. This site is intended for US Healthcare Professionals only.
Other product/company names mentioned herein are the trademarks of their respective owners.
The information contained in LillyOncologyPipeline.com is technical in nature and intended for Healthcare Professionals in the United States only. If you are a US Healthcare Professional, click the “Continue” button below.
Yes, I am a US Healthcare Professional and would like to continue.