BTK Inhibitor
Pirtobrutinib
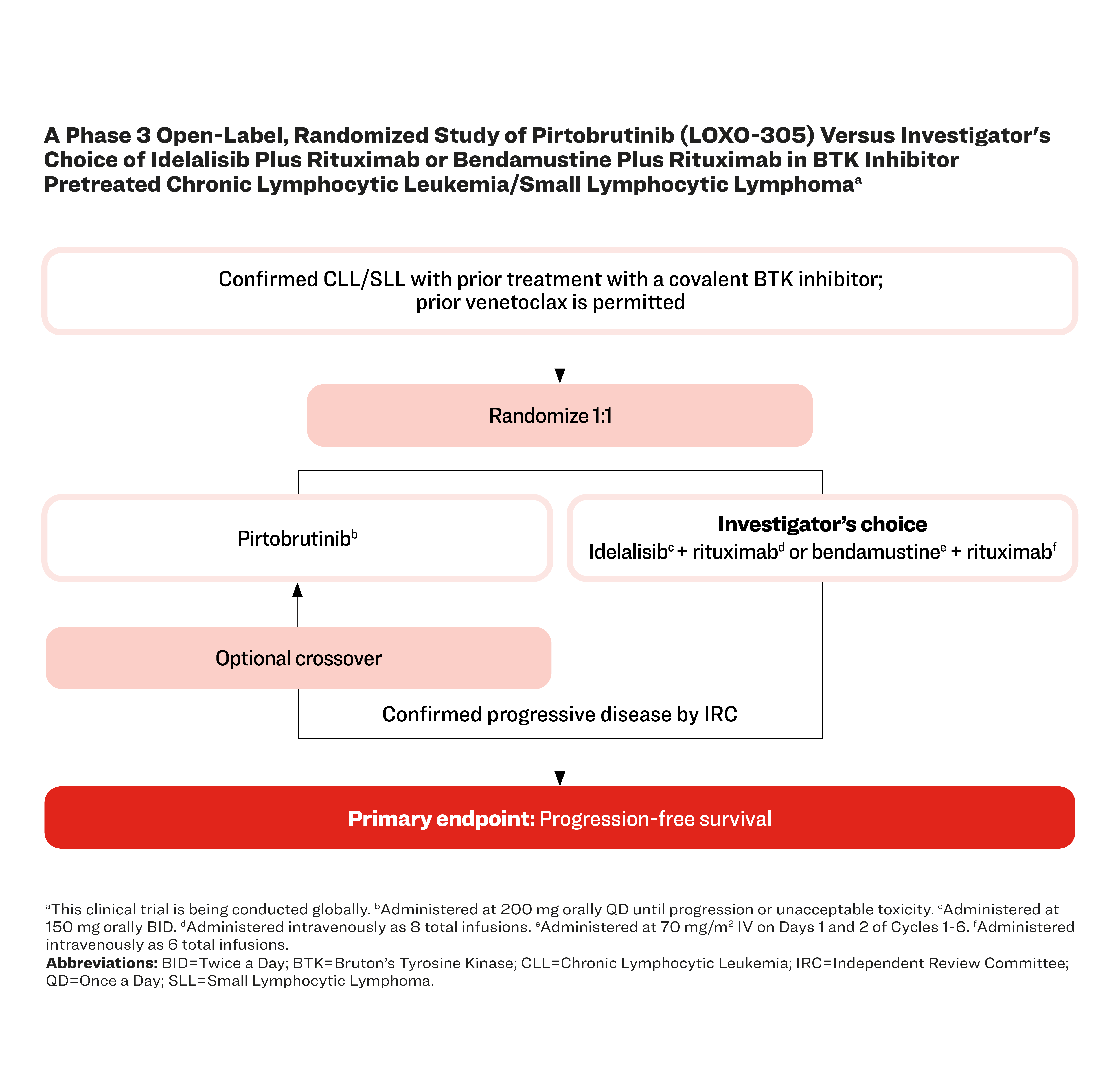
A Phase 3 Open-Label, Randomized Study of Pirtobrutinib (LOXO-305) Versus Investigator's Choice of Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in BTK Inhibitor Pretreated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphomaa
Related Resources: