BTK Inhibitor
Pirtobrutinib
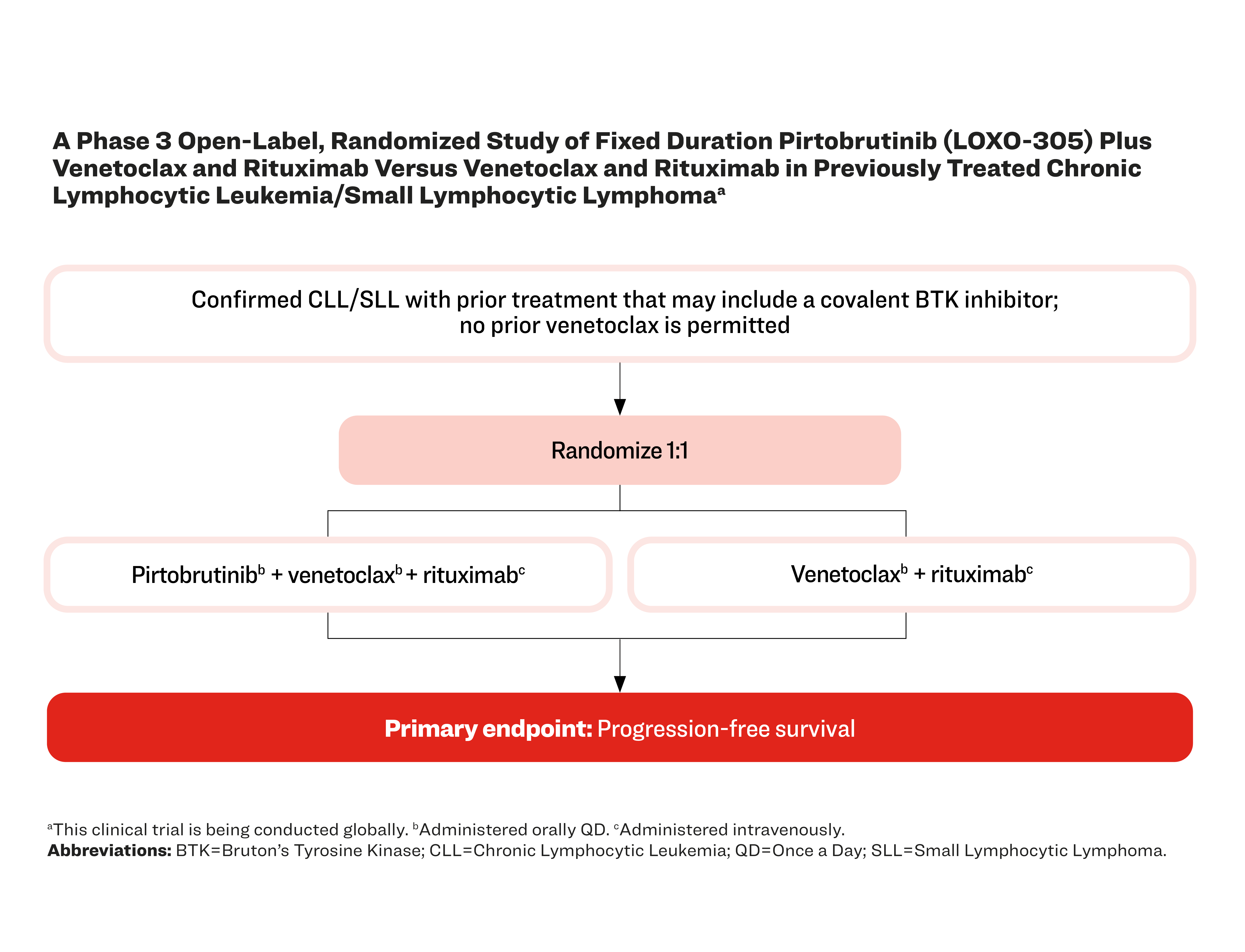
A Phase 3 Open-Label, Randomized Study of Fixed Duration Pirtobrutinib (LOXO-305) Plus Venetoclax and Rituximab Versus Venetoclax and Rituximab in Previously Treated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphomaa
Related Resources: