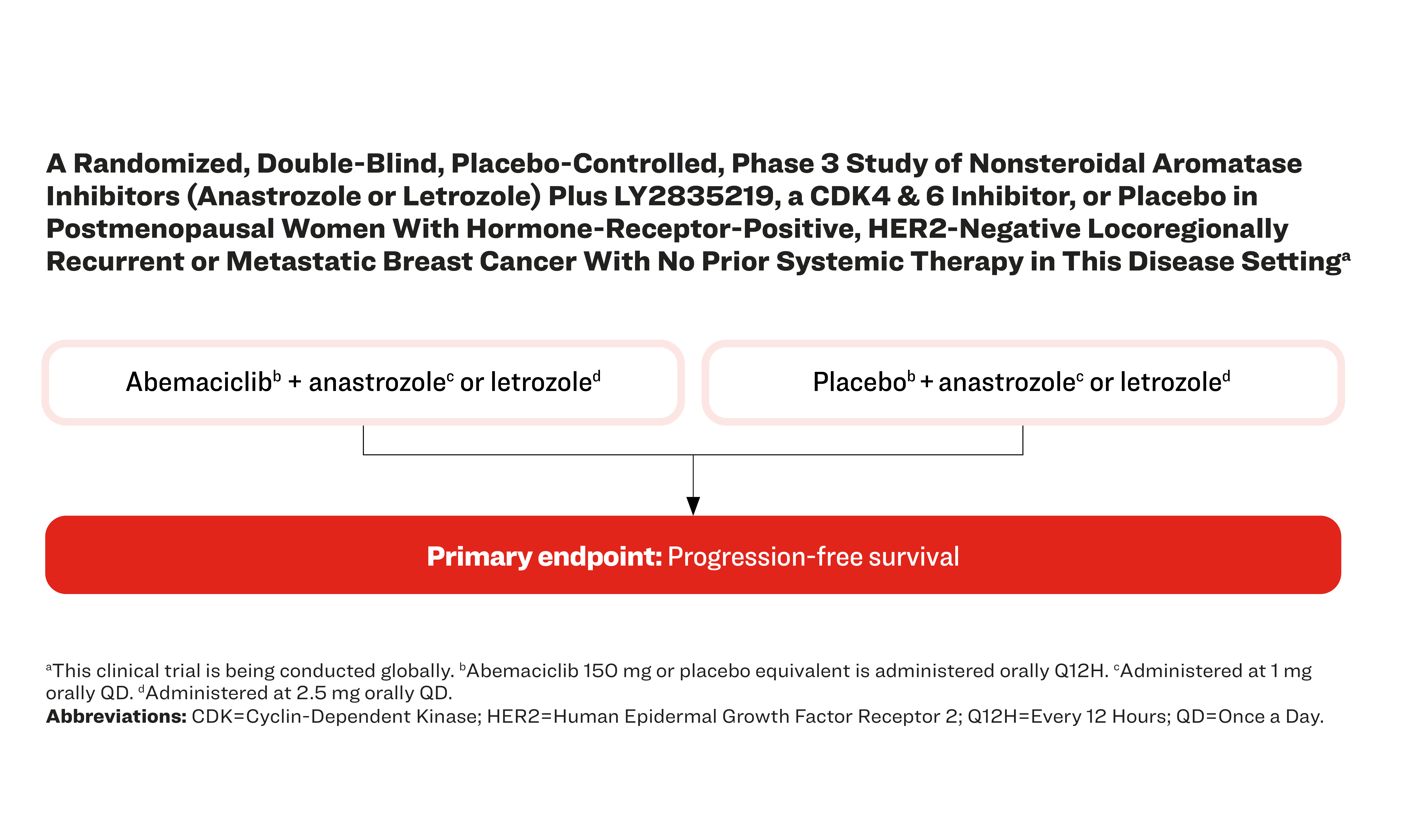
A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study of Nonsteroidal Aromatase Inhibitors (Anastrozole or Letrozole) Plus LY2835219, a CDK4 & 6 Inhibitor, or Placebo in Postmenopausal Women With Hormone-Receptor-Positive, HER2-Negative Locoregionally Recurrent or Metastatic Breast Cancer With No Prior Systemic Therapy in This Disease Settinga