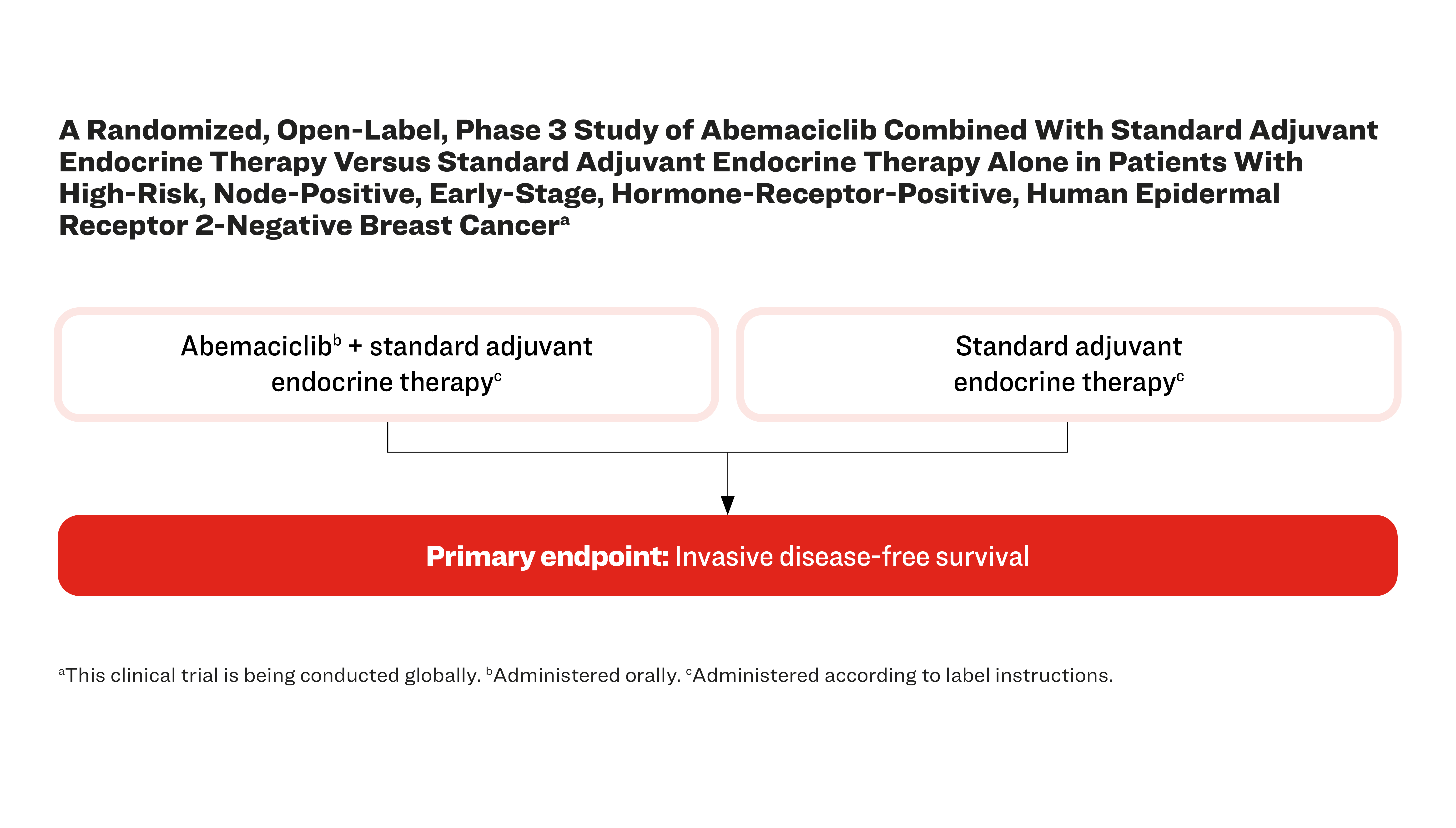
A Randomized, Open-Label, Phase 3 Study of Abemaciclib Combined With Standard Adjuvant Endocrine Therapy Versus Standard Adjuvant Endocrine Therapy Alone in Patients With High-Risk, Node-Positive, Early-Stage, Hormone-Receptor-Positive, Human Epidermal Receptor 2-Negative Breast Cancera