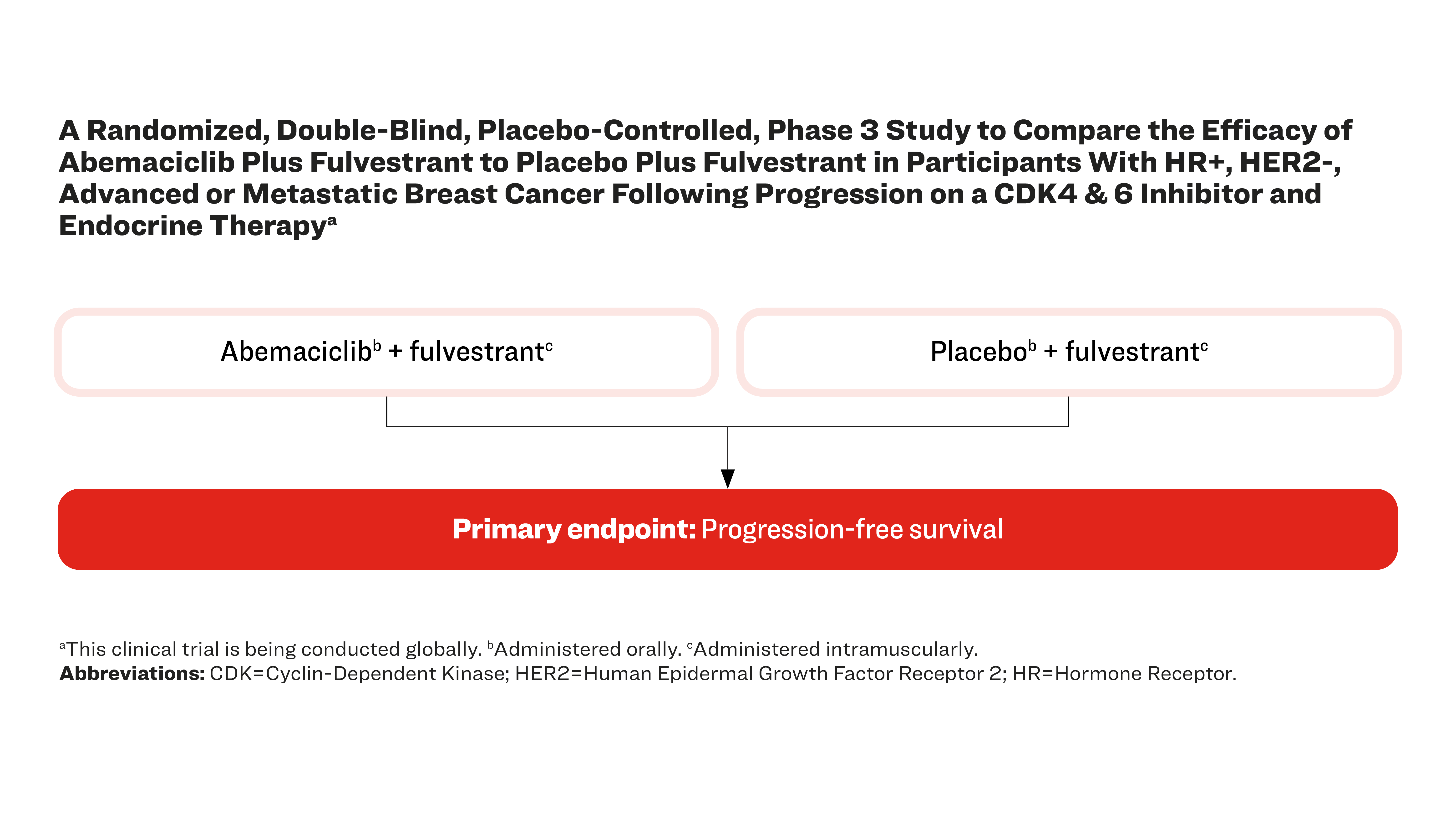
A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Compare the Efficacy of Abemaciclib Plus Fulvestrant to Placebo Plus Fulvestrant in Participants With HR+, HER2-, Advanced or Metastatic Breast Cancer Following Progression on a CDK4 & 6 Inhibitor and Endocrine Therapya