CDK4/6 Inhibitor
Abemaciclib
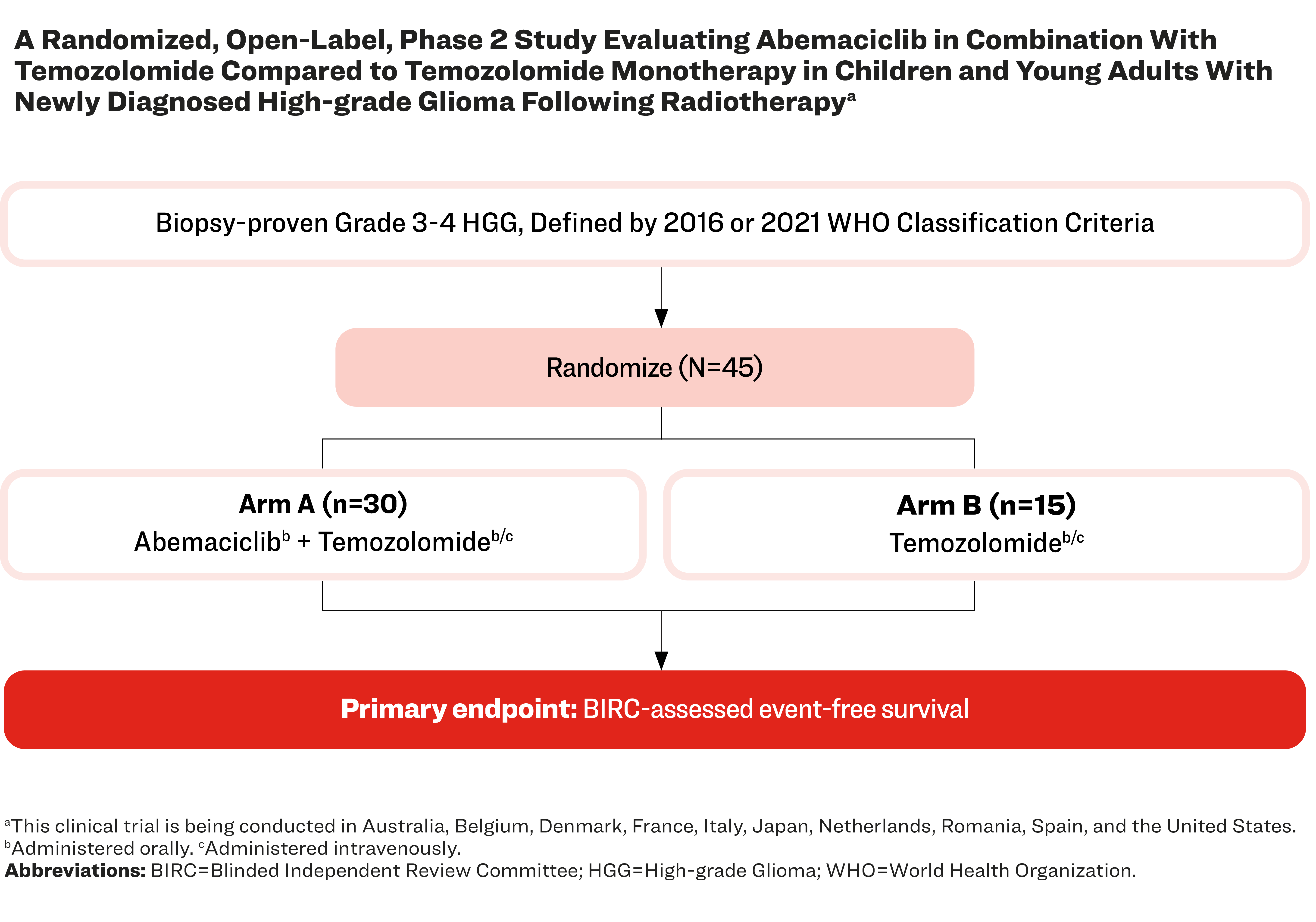
A Randomized, Open-Label, Phase 2 Study Evaluating Abemaciclib in Combination With Temozolomide Compared to Temozolomide Monotherapy in Children and Young Adults With Newly Diagnosed High-Grade Glioma Following Radiotherapya
Related Resources: