KRAS G12C Inhibitor
Olomorasib
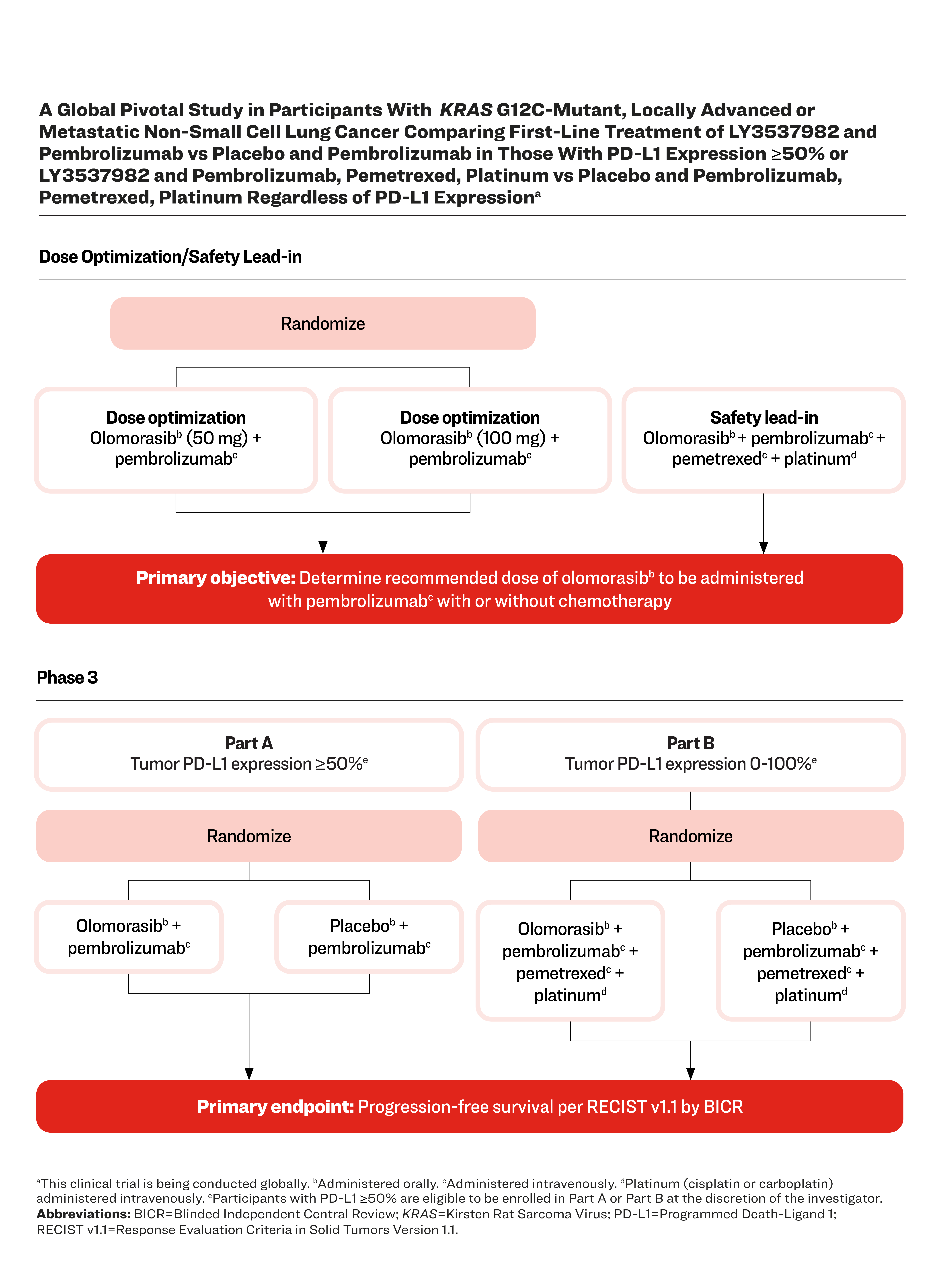
A Global Pivotal Study in Participants With KRAS G12C-Mutant, Locally Advanced or Metastatic Non-Small Cell Lung Cancer Comparing First-Line Treatment of LY3537982 and Pembrolizumab vs Placebo and Pembrolizumab in Those With PD-L1 Expression ≥50% or LY3537982 and Pembrolizumab, Pemetrexed, Platinum vs Placebo and Pembrolizumab, Pemetrexed, Platinum Regardless of PD-L1 Expressiona
Related Resources: