RET Inhibitor
Selpercatinib
A Phase 1/2 Study of the Oral RET Inhibitor Selpercatinib in Pediatric Patients With Advanced RET-Altered Solid or Primary Central Nervous System Tumorsa
Related Resources:
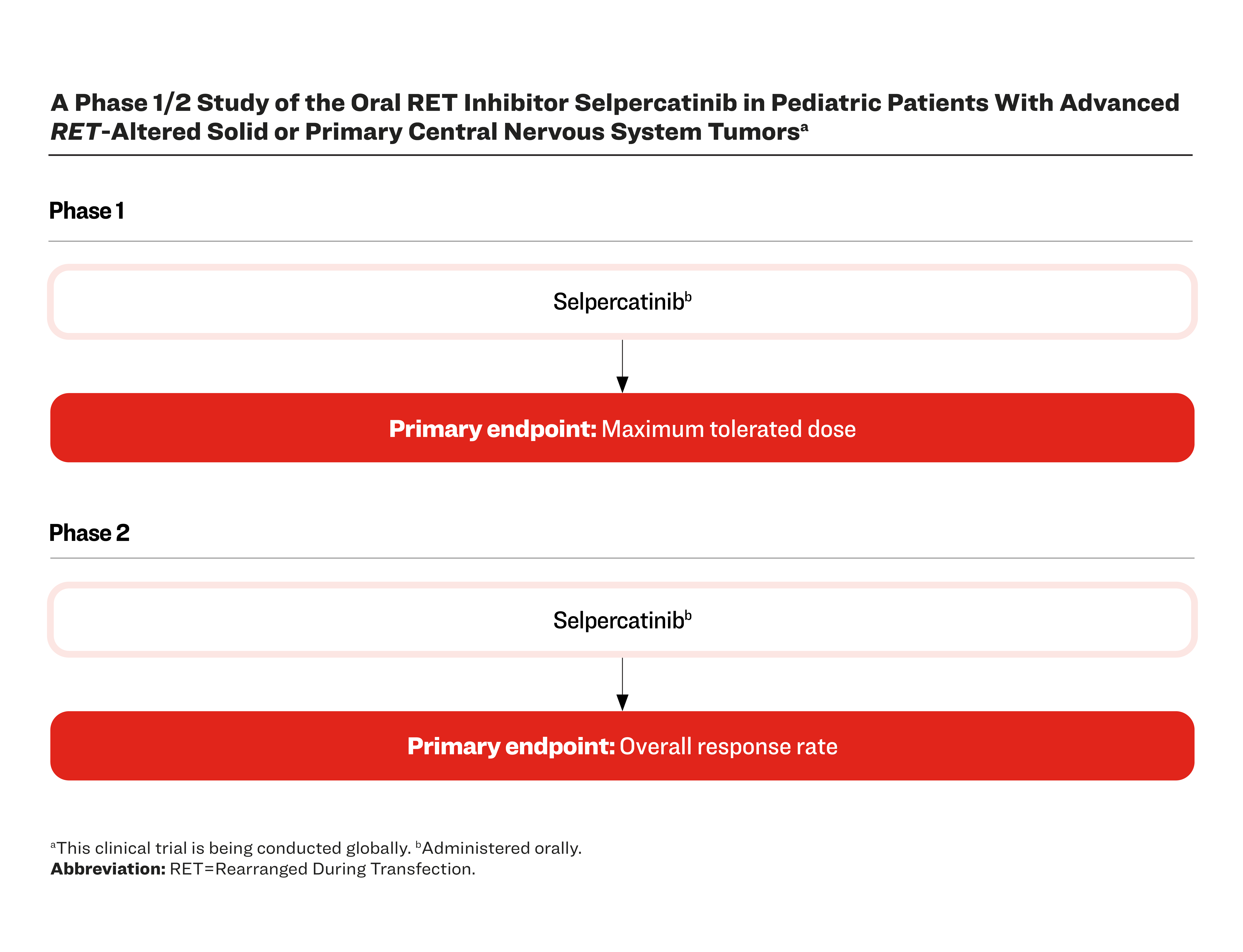
A Phase 1/2 Study of the Oral RET Inhibitor Selpercatinib in Pediatric Patients With Advanced RET-Altered Solid or Primary Central Nervous System Tumorsa

or visit www.clinicaltrials.gov for more information on this trial