Selective Estrogen Receptor Degrader
Imlunestrant
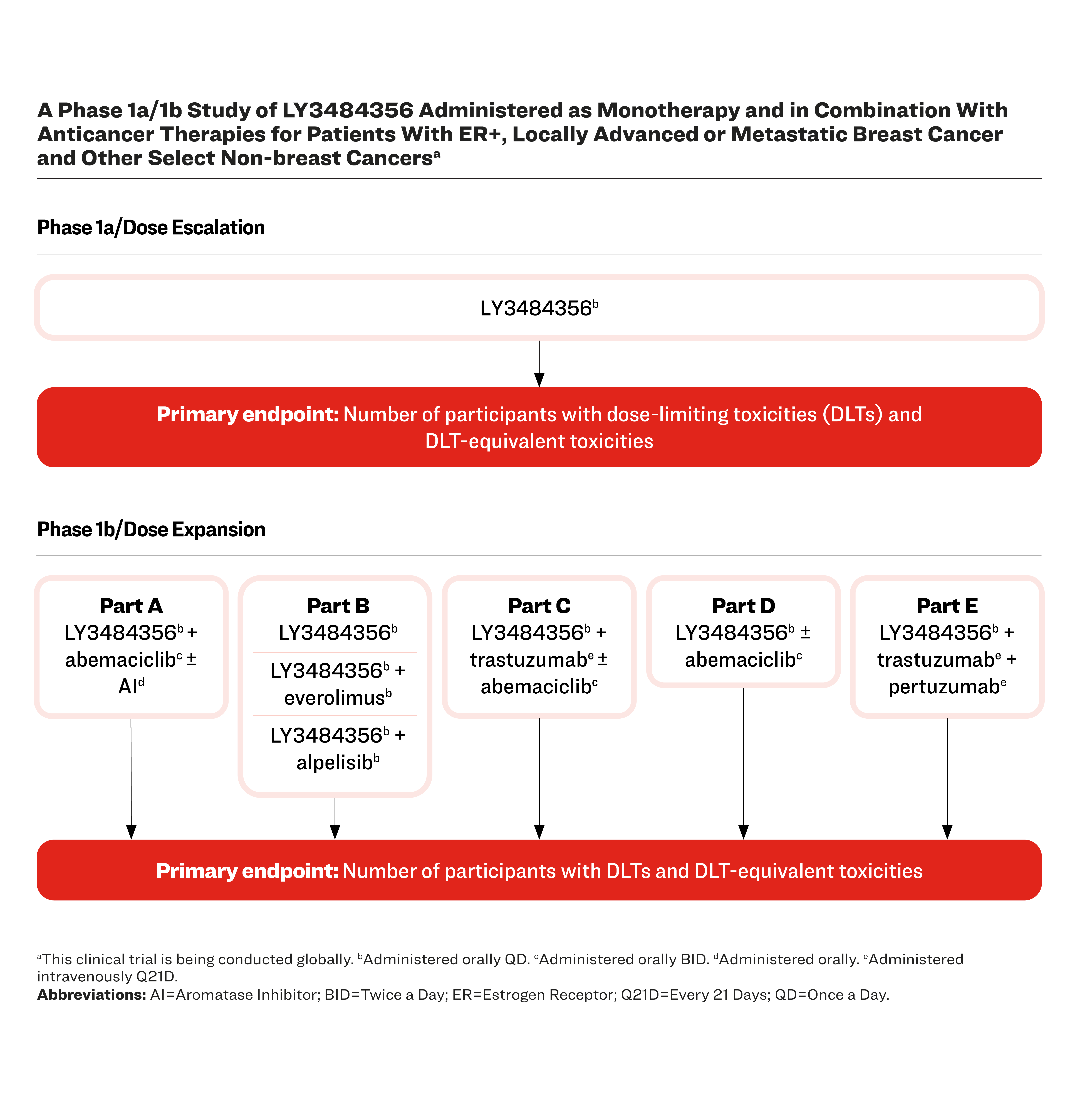
A Phase 1a/1b Study of LY3484356 Administered as Monotherapy and in Combination With Anticancer Therapies for Patients With ER+, Locally Advanced or Metastatic Breast Cancer and Other Select Non-breast Cancersa
Related Resources: