Selective Estrogen Receptor Degrader
Imlunestrant
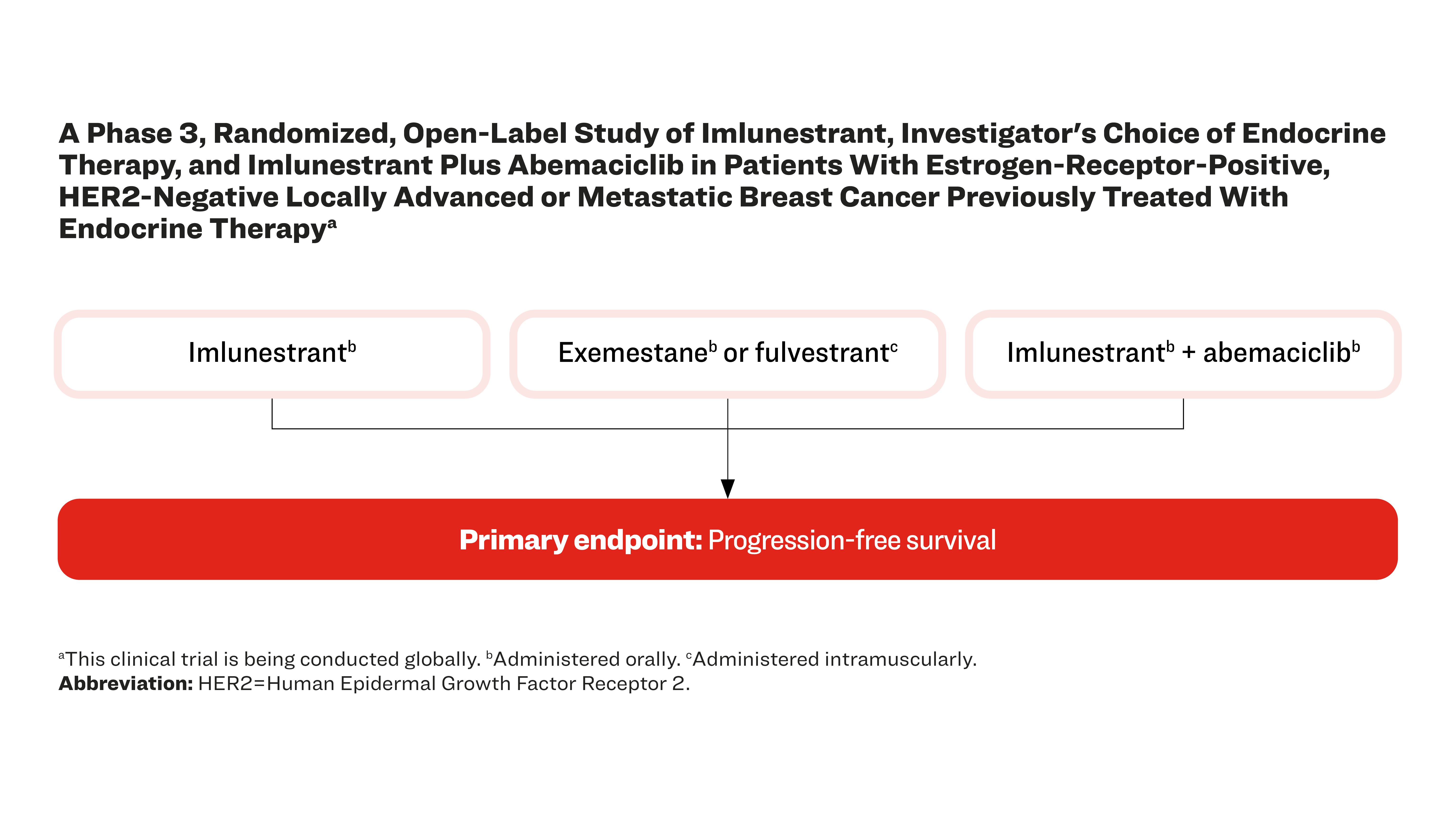
A Phase 3, Randomized, Open-Label Study of Imlunestrant, Investigator's Choice of Endocrine Therapy, and Imlunestrant Plus Abemaciclib in Patients With Estrogen-Receptor-Positive, HER2-Negative Locally Advanced or Metastatic Breast Cancer Previously Treated With Endocrine Therapya
Related Resources: