Selective Estrogen Receptor Degrader
Imlunestrant
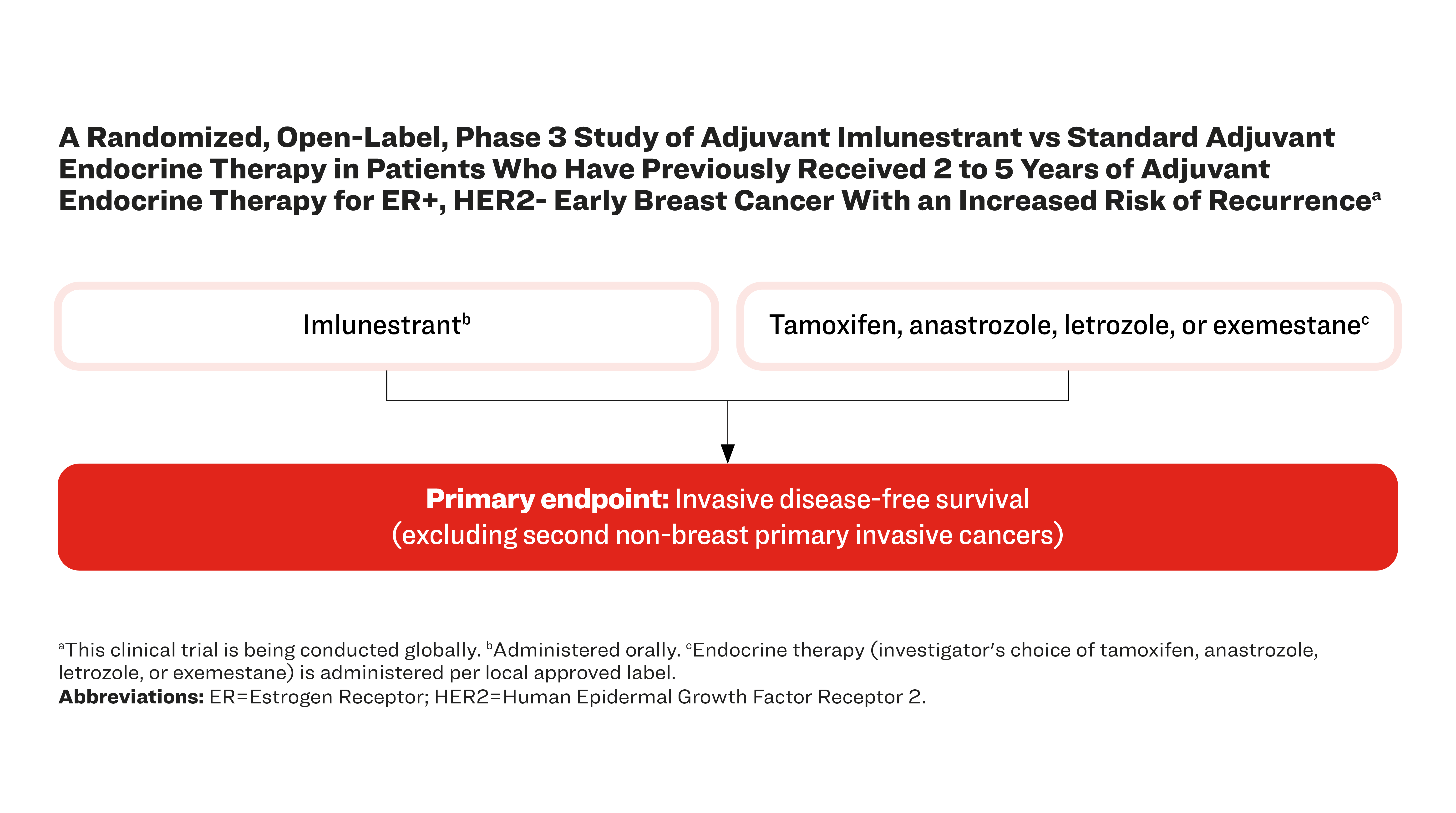
A Randomized, Open-Label, Phase 3 Study of Adjuvant Imlunestrant vs Standard Adjuvant Endocrine Therapy in Patients Who Have Previously Received 2 to 5 Years of Adjuvant Endocrine Therapy for ER+, HER2- Early Breast Cancer With an Increased Risk of Recurrencea
Related Resources: