PIPELINE > TRIAL OVERVIEW
KRAS G12D Inhibitor
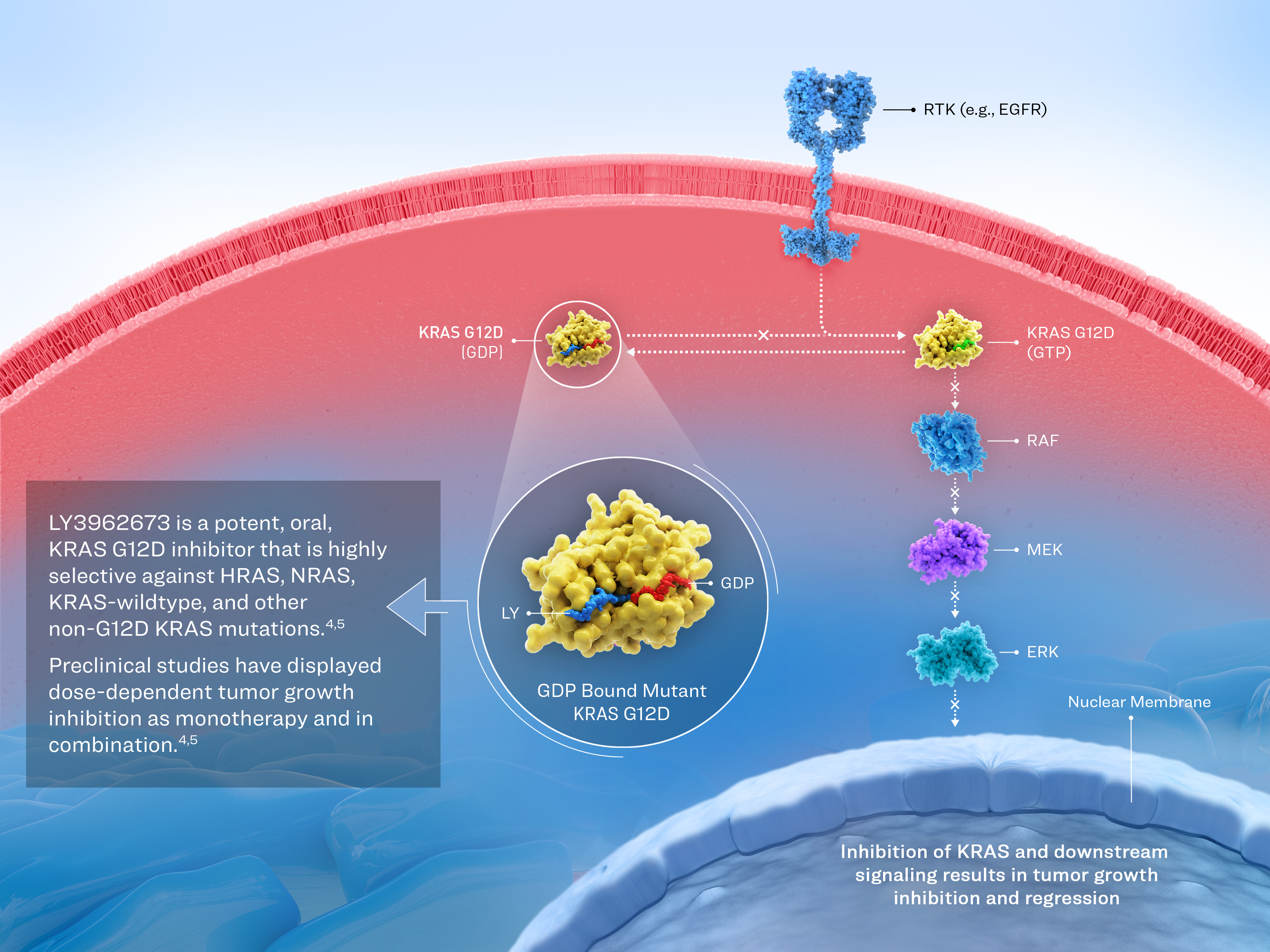
LY3962673
Kano Y1; Hofmann MH2; Ostrem JML and Shokat KM3; Gong X4; Iyer C5
Target
Molecule
Clinical Development
References
- Kano Y, et al. Nat Comm. 2019;10(1):224.
- Hofmann MH, et al. Cancer Discov. 2022;12(4):924-937.
- Ostrem JML, Shokat KM. Nat Rev Drug Discov. 2016;15(11):771-785.
- Gong X, et al. LY3962673, an oral, highly potent, mutant-selective, and non-covalent KRAS G12D inhibitor demonstrates robust anti-tumor activity in KRAS G12D models. Presented at: AACR Annual Meeting; April 5-10, 2024; San Diego, CA. Poster #3316.
- Iyer C, et al. Preclinical characterization of LY3962673, an orally bioavailable, highly potent, and selective KRAS G12D inhibitor. Presented at: AACR-NCI-EORTC Annual Meeting; October 11-15, 2023; Boston, MA. Poster B115.
- Kim D, et al. Nature. 2023;619(7968):160-166.
- Bournet B, et al. Clin Transl Gastroenterol. 2016;7(3):e157.
- Ricciuti B, et al. Ann Oncol. 2022;33(10):1029-1040.
A Phase 1a/1b Trial of LY3962673 in Participants With KRAS G12D-Mutant Solid Tumors. ClinicalTrials.gov identifier: NCT06586515. Updated November 18, 2024. Accessed November 20, 2024. https://clinicaltrials.gov/study/NCT06586515
For information on trial enrollment, locations, and more, call
1-800-545-5979.
or visit www.clinicaltrials.gov for more information on this trial

