KRAS G12D Inhibitor
LY3962673
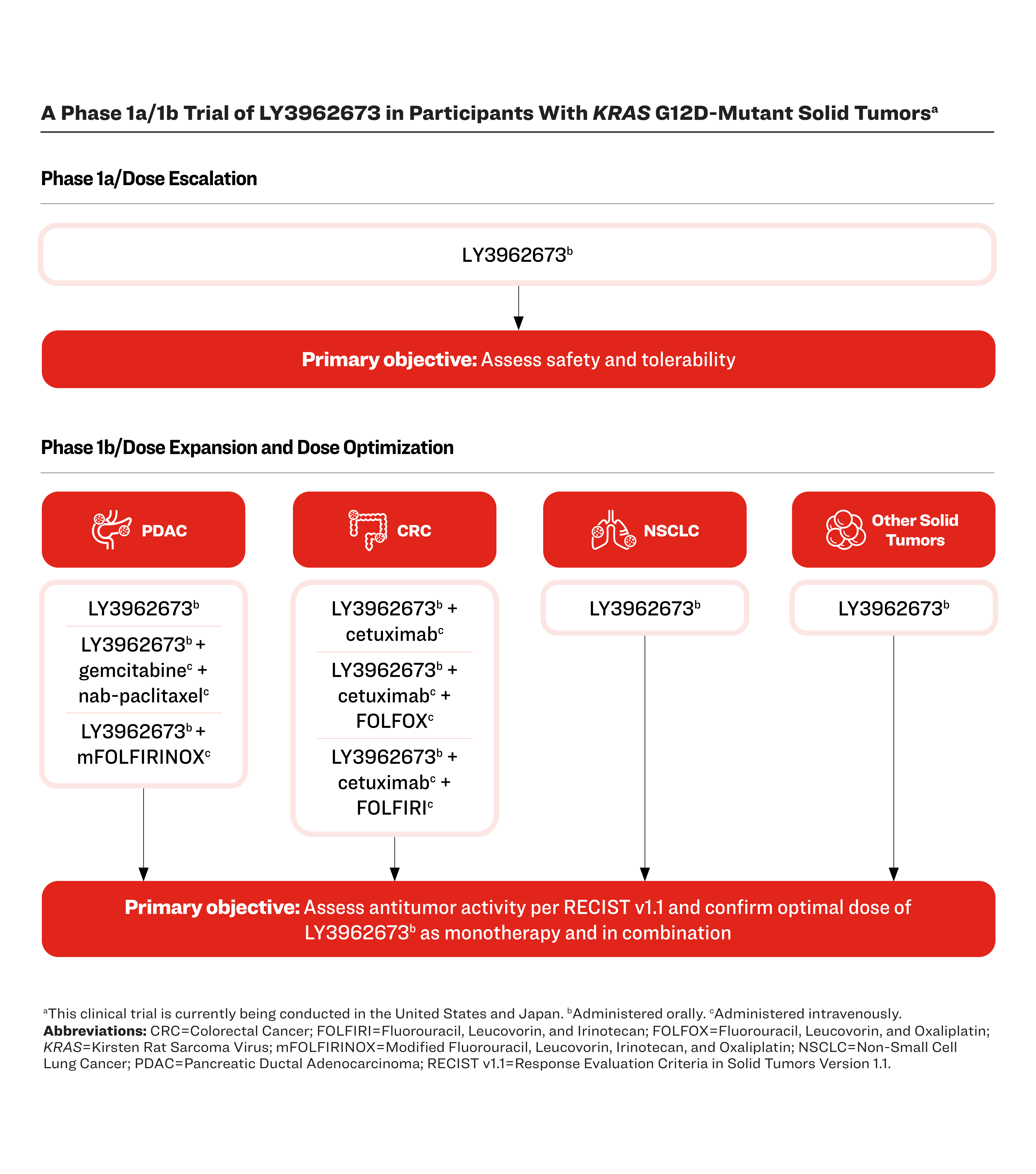
A Phase 1a/1b Trial of LY3962673 in Participants With KRAS G12D-Mutant Solid Tumorsa
Related Resources:
Key Inclusion Criteria
Key Exclusion Criteria
This clinical trial is currently being conducted in the United States and Japan.
Administered orally.
Administered intravenously.


